EKSO GT
INTRODUCING Ekso GT: A REVOLUTIONARY DEVICE BRINGING MOBILITY BACK TO STROKE AND SPINAL CORD INJURY PATIENTS
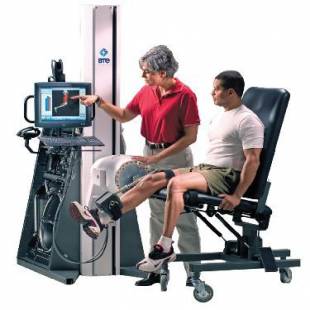
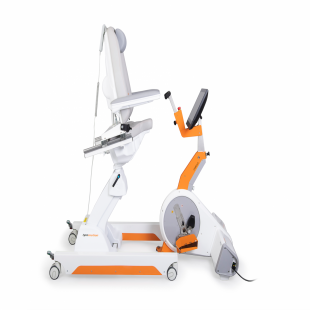
Ekso™ GT is the first FDA-cleared exoskeleton cleared for use with stroke, and spinal cord injury levels to C7.
The Ekso GT with smart Variable Assist™ (SmartAssist) software is the only exoskeleton available for rehabilitation institutions that can provide adaptive amounts of power to either side of the patient’s body, challenging the patient as they progress through their continuum of care.
The suit’s patented technology provides the ability to mobilize patients earlier, more frequently and with a greater number of high intensity steps. To date, this device has helped patients take more than 41 million steps in over 115 rehabilitation institutions around the world.)”